Cancer remains a global health challenge, with approximately 20 million new cases and 9.7 million deaths reported in 2022 by the International Agency for Research on Cancer. Two exciting new studies published in the Journal of the American Chemical Society are shedding light on an enzyme called beta-1,3-galactosyltransferase 5 (β3GalT5), which plays a critical role in producing sugar molecules tied to cancer progression. These molecules, known as globo-series glycosphingolipids—including SSEA-3, SSEA-4, and Globo-H—are increasingly recognized for their roles in promoting tumor growth and metastasis. When β3GalT5 gets activated, it fuels the production of these cancer-linked sugars, thus making β3GalT5 a promising target for developing new therapies and vaccines.
Led by Dr. Che Ma and Dr. Chi-Huey Wong at the Genomics Research Center, Academia Sinica, the research teams investigated the structure, mechanism, specificity and synthetic utility of β3GalT5. Using a combination of established and specialized techniques, these studies provide critical insights into the enzyme’s mechanism and therapeutic potential. The works are highlighted in the American Chemical Society press news on March 25 (‘Low-sugar’ vaccine can provide broad immunity against coronavirus variants) and published in this issue of C&EN News.
In the first study, titled "Structure-Based Mechanism and Specificity of Human Galactosyltransferase β3GalT5," researchers used X-ray crystallography to map the enzyme’s three-dimensional structure across its catalytic cycle. The findings detail how β3GalT5 recognizes substrates, transfers sugar molecules, and releases products. The team identified an SN2-like catalytic mechanism with strong acceptors, where the acceptor’s hydroxyl group drives glycosidic bond formation. In the absence of strong acceptors, β3GalT5 slowly hydrolyzes galactose from UDP-Gal via SN1-like mechanisms, involving an oxocarbenium-like intermediate. Notably, the enzyme displays broad substrate specificity, modifying diverse acceptor molecules—a trait that complicates but also enriches its potential as a drug target. These mechanistic insights lay the groundwork for designing inhibitors to block β3GalT5 activity.
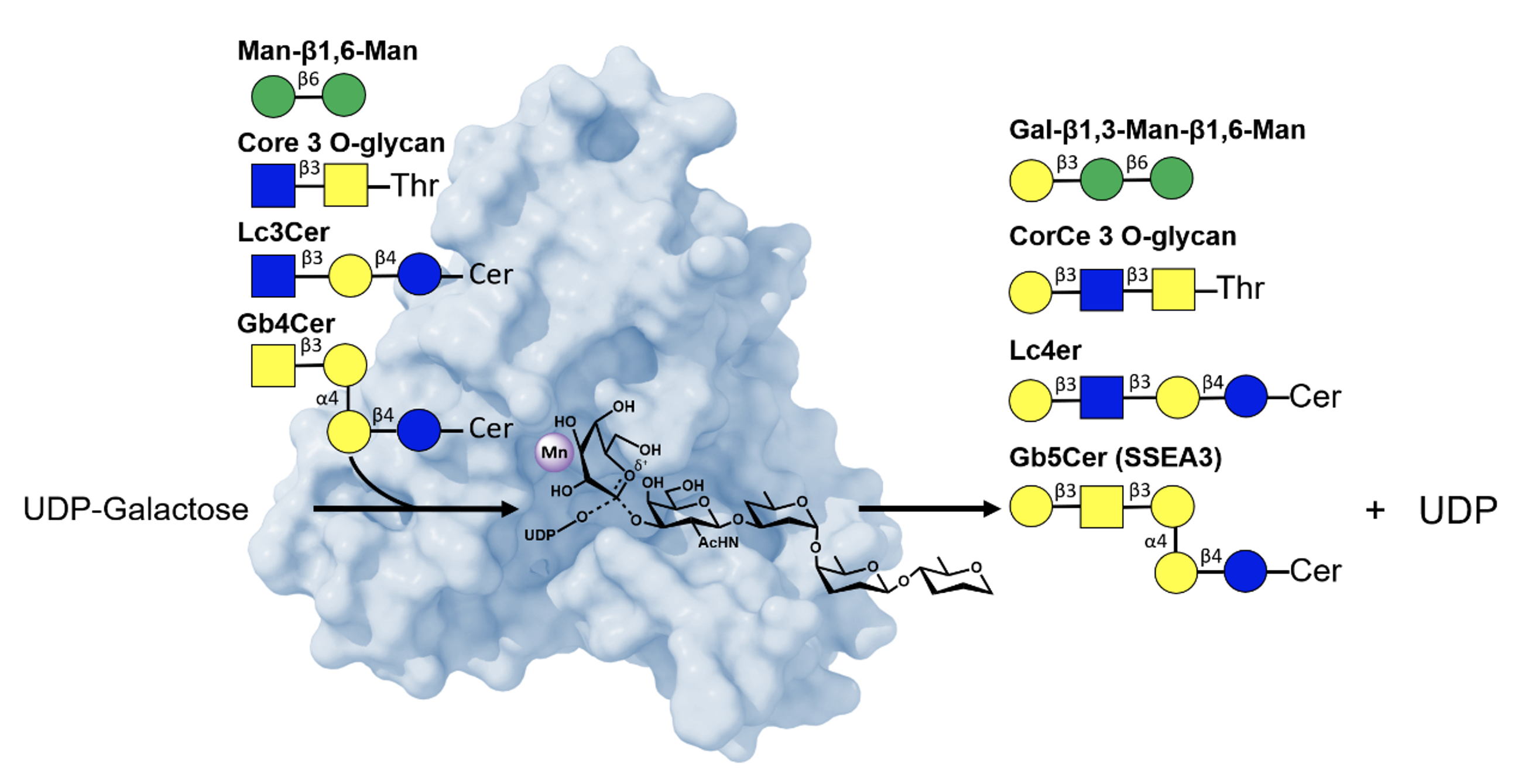 |
| Figure 1. Structural basis of β3GalT5's mechanism and broad acceptor specificity |
The companion study, "Expression of Human β3GalT5-1 in Insect Cells as Active Glycoforms for the Efficient Synthesis of Cancer-Associated Globo-Series Glycans," took a hands-on approach. The team compared the enzyme’s two isozymes, finding β3GalT5-1 significantly more active than β3GalT5-2 in breast cancer cells and both require glycosylation at three N-glycosites for activity, as confirmed using targeted probes and dicer-substrate siRNA as well as enzymatic assay. By expressing the soluble domain of β3GalT5-1 in insect cells and combining a site-specific alanine scan from 3D structure, they identified the S66A variant with ten times the efficiency at making cancer-related sugars like Globo-H. This breakthrough could make it easier and cheaper to produce Globo-H vaccines, which are currently in late-stage clinical trials for cancers like triple-negative breast cancer, drug-resistant lung cancer, and esophageal cancer.
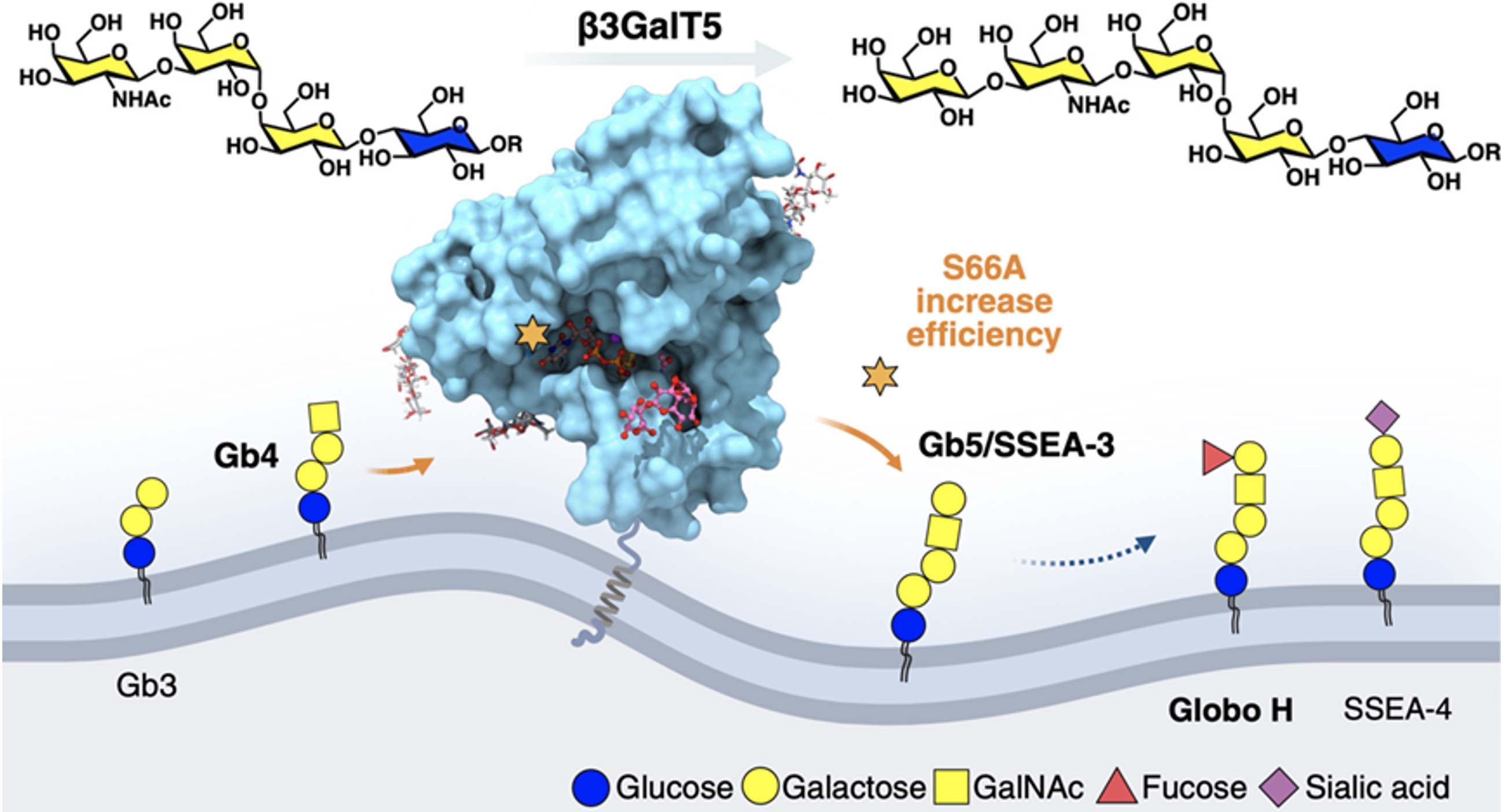 |
| Figure 2. S66A β3GalT5 enhance the catalytic efficiency in synthesizing globo-series glycans. |
Together, the synergy of structural biology and enzyme engineering exemplifies how fundamental research can translate into real-world impact. These discoveries highlight β3GalT5 as a promising target for drug development and a tool for producing Globo-series glycans, advancing cancer immunotherapy and treatment strategies. The studies, co-first authored by Dr. Jennifer Lo and Mr. Chih-Chuan Kung, are available online at: